Record display
 PROTA4U Homepage
PROTA4U Homepage Select translation pop-up: Select translation pop-up: |
 |
PROTA4U Record display |
 PROTA4U Homepage PROTA4U Homepage
|
| Sp. pl. 2: 1188 (1753). | |||
| show more data (9) | comments (0) |
| Arecaceae (Palmae) | |||
| show more data (19) | comments (0) |
| 2n = 32 | |||
| show more data (7) | comments (0) |
| show more data (10) | comments (0) |
| Coconut palm (En). Cocotier (Fr). Coqueiro (Po). Mnazi (Sw). | |||
| show more data (10) | comments (0) |
| Cocos nucifera is native to the coastal regions of tropical Asia and the Pacific, but its primary centre of origin is the subject of speculation. Fossil coconuts have been found as far apart as India and New Zealand. The ability of the thickly husked and slow germinating fruit of wild coconut palm to remain viable after floating long distances at sea ensured wide natural dispersal in the Indo-Pacific region long before domestication may have started in South-East Asia. The domesticated coconut palm has a robust stem and large fruits, which cannot survive long periods of floating at sea because of thinner husks and shells and quicker germination. Initial dissemination of the domesticated coconut palm coincided with migrations of South-East Asian peoples to the Pacific and India, which started 3000 years ago. Where wild coconut palms already occurred, there was opportunity for introgression with domesticated types, as they remained compatible. Polynesian, Malay and Arab navigators played an important role in further dispersal of coconut into the Pacific, Asia and East Africa. Coconut palm became truly pantropical in the 16th century after European explorers had taken it to West Africa, the Caribbean and the Atlantic coast of tropical America. Coconut palm is planted throughout lowland tropical Africa, mainly along the coast in more humid areas. | |||
| show more data (47) | comments (0) |
| Coconut palm has been called the ‘tree of life’, because of its value as provider of so many useful products. For domestic oil extraction a mixture of grated fresh endosperm from the fruit and water is boiled until floating oil can be skimmed off. For industrial production the endosperm is first dried to copra before it is taken to the mill for oil extraction. High-grade oil is used for cooking or in the manufacture of margarine, shortening, filled milk, ice cream and confectioneries. Oil of low grades is processed into soap, detergents, cosmetics, shampoos, paints, varnishes and pharmaceutical products. Remnant fatty acids and alcohols and their methyl esters find application as components of emulsifiers and surfactants. The press cake or copra meal is a good livestock feed. Coconut milk or cream pressed from the mix of freshly grated endosperm with water has been a traditional ingredient in many African and especially Asian food and bakery products. It is now also marketed in pasteurized and homogenized canned or powdered form. Skimmed milk powder, produced after boiling fresh coconut milk and removing the floating oil, contains 25% hydrolyzed starch and can be mixed with water to make a beverage. Protein can be separated by ultrafiltration and spray-dried into a white powder, which is very suitable for infant nutrition. Shredded or thinly sliced and desiccated fresh coconut endosperm is a favourite side dish and ingredient in many confectionery, bakery and snack food products. Water in the cavity of young coconuts provides a cool and sweet-tasting, popular refreshment. It is now also commercially preserved without altering its typical flavour. The tender, jelly-like endosperm of young coconuts is a delicacy consumed directly or grated and mixed with food. The haustorium or apple which fills the cavity of germinating coconuts is also edible. The liquid endosperm of mature coconuts can be used to produce a fermented gelatinous dessert called ‘nata de coco’ in the Philippines. The shell (endocarp) covering the seed can be made into household utensils and decorated pots, converted into shell charcoal (suitable for activation) or used as fuel. Finely ground coconut shell is used as filler for resin glues and moulding powders. Green husks (mesocarp) yield, after retting, white coir (yellow fibres) for making ropes, carpets, mats and geo-textiles. Brown coir from husks of mature fruits is used in brushes (long bristle fibres), mattresses, upholstery and particle board (short fibres). Coir dust or coco peat is a component of potting mixtures (water-holding capacity of 700–900%), light building materials, thermal insulation, adhesives and binders. A sweet sap containing about 15% sucrose is tapped from unopened inflorescences. It is a refreshing toddy when consumed fresh and it transforms into a light alcoholic wine when fermented. A by-product of palm wine is vinegar. Boiling fresh sap yields palm syrup and sugar. Distillation of palm wine yields a potent alcoholic beverage called ‘arak’. The leaves are used to thatch roofs; the leaflets are plaited into mats, baskets, bags and hats; immature leaflets are made into traditional decorations and small bags or containers for food; the midribs of the leaflets are formed into brooms. The palm heart, which consists of the white, tender tissues of the youngest, unopened leaves at the stem apex, is considered a delicacy. Young coconut palms (3–4 years) have the heaviest palm heart, weighing 6–12 kg. The wood of old palms is very hard, but a freshly felled trunk can be sawn with a special tungsten carbide-tipped saw blade. Preservative treatment of sawn wood is needed if it is to be used for construction or any outdoor use. Coconut palm wood is also suitable for furniture, household utensils and tool handles. Medicinal uses have been attributed to coconut palm. The roots are considered antipyretic and diuretic. Milk of young coconut is diuretic, laxative, antidiarrhoeic and counteracts the effects of poison. The oil is used to treat diseased skin and teeth and mixed with other medicines to make embrocations. Coconut palm has also an ornamental value. The palms’ often-slanting stems and graceful crowns bordering a white beach along a blue sea are hallmarks of the tropics. | |||
| show more data (22) | comments (0) |
| Average annual world production in 2002–2004 was estimated at about 58,000 million coconuts, equivalent to 10.5 million t of copra, from 11.8 million ha in 93 countries. The copra of about 55% of all coconuts is commercially extracted to produce annually some 3.3 million t oil and 1.8 million t coconut meal, the remaining coconuts being processed domestically or sold as young coconuts for drinking. Coconut palm is mainly a smallholder crop and only about 6% of the total area consist of estates. Asia and the Pacific account for 86% of world production, Latin America and the Caribbean for 10% and Africa for 3% (6% of planted area). The major producers are Indonesia (30% of world production), the Philippines (23%) and India (17%). Estimated areas planted with coconut palm in Africa are: Tanzania 310,000 ha, Mozambique 70,000 ha, Ghana 55,000 ha, Nigeria 50,000 ha, Madagascar 33,000 ha and Côte d’Ivoire 30,000 ha. With about 2.1 million t of oil traded annually, coconut palm is the 7th most important supplier of vegetable oil in the global market. It has a special position in the market together with palm-kernel oil as a major source of lauric oil. The Philippines exports about 80% of their national coconut oil production in contrast to Indonesia, which exports only 20–30%, and India, which exports almost none. About 50% of the coconut meal produced annually in the world is exported; about 500,000 t by the Philippines and 300,000 t by Indonesia. | |||
| show more data (3) | comments (0) |
| Fresh, mature fruits weigh 1.1–2.5 kg and consist of husk (exocarp and mesocarp) 30–45%, shell (endocarp) 14–16%, endosperm 25–33% and free water in the cavity 13–25%. Fresh endosperm contains 35–52% water; high-quality copra has 63–68% oil, no more than 6% water and less than 1% free fatty acid. The proximate composition of dried copra per 100 g edible portion is: water 3 g, energy 2761 kJ (660 kcal), protein 7 g, fat 65 g, carbohydrate 24 g, fibre 16 g, Ca 26 mg, Mg 90 mg, P 206 mg, Fe 3 mg, Zn 2 mg, vitamin A 0 mg, thiamin 0.06 mg, riboflavin 0.1 mg, niacin 0.6 mg, vitamin B6 0.3 mg, folate 0 mg, ascorbic acid 1.5 mg. The fatty acid composition of coconut oil is: caproic acid 0.6%, caprylic acid 7.5%, capric acid 6%, lauric acid 45%, myristic acid 17%, palmitic acid 8%, stearic acid 3%, oleic acid 6%, linoleic acid 2% (USDA, 2006). More than 90% of the fatty acids are saturated. Lauric acid is an easily digestible source of energy and a precursor of the antimicrobial lipid mono-laurin, which enhances the human immune system. It is hardly deposited at all in body tissues. Coconut milk contains approximately: fat 15–35%, protein 3% and carbohydrate 2%; powdered coconut milk: fat 60%, protein 7% and carbohydrate 27%; dried and powdered skim milk: fat 6%, protein 24% and carbohydrate 25%; spray-dried coconut protein powder: protein 59%. Presscake contains: fat 6%, protein 21%, carbohydrate 49% and crude fibre 12%. Coconut palm wood, known as ‘cocowood’ in trade, has a basic density of 400–600 kg/m3, the basal annular outer parts as much as 850 kg/m3. It is suitable as timber for construction purposes because of its moderate to high strength and lack of knots. | |||
| show more data (2) | comments (0) |
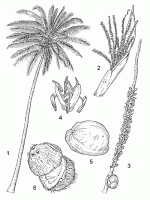
| An unbranched palm tree up to 30 m tall, with a terminal crown of leaves; roots mostly in the top 1.5 m of soil, normally c. 6 m × 1 cm but in optimum soil conditions up to 30 m long; stem cylindrical, erect, often curved or slanting, up to 40 cm in diameter but the swollen base up to 60 cm, pale grey, conspicuously ringed with scars of fallen leaves. Leaves arranged spirally, pinnately compound, 4.5–6(–7) m long, 25–35 unfolded leaves per plant; petiole stout with clasping, fibrous sheath at base, about one quarter of total leaf length, grooved above, rounded beneath; leaflets 200–250, linear-lanceolate, 50–120 cm × 1.5–5 cm, single folded lengthwise at base, apex acute, regularly arranged in one plane. Inflorescence 1–2 m long, in axils of leaves, enclosed by a large bract when young, consisting of up to 40 spirally arranged spikes, each bearing 200–300 male flowers and 1–few female flowers in basal parts. Flowers unisexual, regular, 3-merous; male flowers 1–3 together, sessile, 0.5–1.5 cm in diameter, pale yellow, with 3 small sepals, 3 larger petals, 6 stamens in 2 whorls and a rudimentary pistil; female flowers solitary, much larger than male flowers, globose in bud, ovoid at anthesis, 2–3 cm in diameter, enveloped by 2 small scaly bracteoles, sepals and petals each 3, almost orbicular, almost equal, persistent and enlarging in fruit, with large 3-celled ovary, 3 sessile triangular stigmas and 3 nectaries near ovary base. Fruit a globose, ovoid or ellipsoid drupe, indistinctly 3-angled, 20–30 cm long, weighing up to 2.5 kg, 1-seeded; exocarp very thin, 0.1 mm thick, smooth, green, brilliant orange, yellow to ivory-coloured when ripe, usually drying to grey-brown in old fruits; mesocarp (‘husk’) fibrous, 4–8 cm thick, pale brown; stone (called ‘nut’) ovoid, 10–15 cm in diameter, endocarp (‘shell’) 3–6 mm thick, hard, stony, dark brown, indistinctly 3-angled with 3 longitudinal ridges and 3 large, slightly sunken pores (‘eyes’) at basal end. Seed with a thin brown testa closely appressed to endocarp and adhering firmly to firm, white, oil-rich endosperm (‘meat’), 1–2 cm thick, embryo 0.5–1 cm long, with large cavity in centre of seed. | |||
| show more data (11) | comments (0) |
| Cocos nucifera is the only species of the genus Cocos. A generally accepted classification system for the wide variability of coconut palm does not exist. Coconut palm types that are thought to be of natural origin are said to be of the ‘Niu kafa type’ (fruits long, angular, with thick mesocarp, floating easily, with long-lasting viability and slow germination); those which are thought to have developed under cultivation are of the ‘Niu vai type’ (fruits globose, with thinner mesocarp, not floating easily, with thick endosperm and earlier germination). ‘Niu kafa’ and ‘Niu vai’ are Polynesian words. Where these 2 types come into contact, introgression takes place. Up to now, cultivated coconut palm has been classified into 2 groups: tall types and dwarf types. More than 95% of all cultivated coconut palms belong to the tall type. Cultivars of the tall type are: ‘Malayan Tall’, ‘Rennell Island Tall’, ‘Vanuatu Tall’, ‘Jamaican Tall’, ‘West African Tall’ and ‘East African Tall’. The dwarf type is rare, but can be found in different ecotypes. Characteristics of the dwarf type are: weaker growth and slow height increment; slender stem with almost no thickened base; smaller leaves, inflorescences and fruits; precocity and rapid succession of inflorescences; high degree of self-pollination. The inheritance of dwarfness is not well understood but hybrids are usually intermediate in height increment and other characteristics to the tall and dwarf parents. Three different types of dwarf cultivars exist: the ‘Niu leka’ from Fiji which differs only from the tall types by its very short internodes and short rigid leaves; the medium-sized types such as ‘Malayan Dwarf’ from Indonesia, ‘Gangabondam’ from India and ‘King’ from Sri Lanka; and the small dwarf cultivars in various countries. Dwarf types are also differentiated based on the colour of leaf petiole of young palms: green, yellow and red (orange or golden). The fruits of ‘Makapuno’ from the Philippines and ‘Kelapa Kopjor’ from Indonesia have endosperm that fills almost the entire seed cavity. The endosperm is soft, has a peculiar taste and is considered a delicacy. The fruits do not germinate, but the embryos can be cultured in vitro. This character may appear in any tall cultivar. | |||
| show more data (24) | comments (0) |
| Mature fruits of most coconut palm cultivars start germinating soon after harvest. The embryo enlarges and the apical part emerges from the shell. At the same time, the cotyledon develops into a haustorium. The primary root emerges from the apical mass, followed by the plumule. As growth continues they emerge at opposite sides through the husk. Shoot emergence occurs about 8 weeks after placing coconuts in a germination bed, and another 5 weeks later the first leaf starts to unfold. The leaves increase in size but remain entire until the seedling has 7–10 leaves, usually after one year’s growth. Subsequent leaves become progressively pinnately compound. Cultivars of the tall type produce about 10 leaves during the first year, those of the dwarf type about 14. In subsequent years, larger and more leaves are formed, until full leaf size is attained and annual production levels off at 12–18 leaves for tall types and hybrids and 20–22 leaves for dwarf types. Since a leaf of a tall coconut palm remains on the tree for about 2.5 years after unfolding, the leaf number in the crown levels off at 30–35 after 6 or 7 years. Leaf initiation until senescence takes about 4 years. The root system consists of adventitious roots numbering 2000–4000 per palm. Decayed roots are replaced regularly; new roots emerge from the upper part of the thickened basal stem. The regular development of both canopy and root system is well adapted to the constant environment of the humid lowland tropics. The long development periods of large organs give the palm a certain inflexibility to short-term stress. Under adverse conditions, flowering and fruiting are mainly affected, leading to smaller inflorescences and fewer female flowers, abortion of inflorescences, reduction in fruit set, nut size and filling, and premature fruit fall and tapering of the stem. Thus, stress affects yield much more than growth. The size of new leaves and roots has been fixed a long time in advance and cannot be adjusted to short-term stress periods. After long-term stress leaf emergence slows down which further reduces yield, since the emergence of inflorescences follows that of the subtending leaves. At the rosette stage, the growing point continues to enlarge until the size of the leaf initials reflects the prevailing growing conditions; then trunk formation starts. At close spacing, height growth increases at the expense of flowering and fruiting. Precocity and yield are positively correlated with annual leaf formation, as an inflorescence appears in the axil of each leaf. Hence, dwarf types yield earlier and more than tall types. First flowering in tall types occurs after 5–7 years, in dwarf types after 2 years, and in dwarf × tall hybrids 3–4 years after germination. Growing conditions have great influence on these aspects. Coconut palm trees can be more than 100 years old, but highest yields are usually obtained at 10–20 years of age for tall types and a few years earlier in dwarf types and hybrids. In coconut plantations in coastal Tanzania yields slowly increase until the trees are 20 years old, yields increase at a faster rate until age 40 and start declining at age 50. It is recommended to replant when the palms are 60–70 years old. During the first phase of anthesis, which lasts 16–22 days, only male flowers open progressively from the top to the base of the upper spikes and down to the lowest spikes. Each male flower opens, sheds its pollen and abscises within 2 days. The first female flower at the top of the spadix becomes receptive about 3 weeks in tall types or 1 week in dwarf types after the enveloping bract has opened, and the stigmas of the last female flower turn brown 5–12 days later. Female flowers are nectariferous and sweet scented. Pollination is both by insects and by wind. Each female flower remains receptive for 2–3 days. Tall types are generally allogamous because the male and female phases do not overlap, while in dwarf types self-pollination is common due to considerable overlap. Self-pollination can also occur when the female phase of one inflorescence overlaps with the male phase of a second inflorescence on the same tree. About 50–70% of the female flowers abort during the first two months due to poor fertilization or physiological causes. Fruits are mature 11–12 months after anthesis, but may not drop until 15 months old. | |||
| show more data (4) | comments (0) |
| Coconut palm is essentially a crop of the humid tropics. It is fairly adaptable with regard to temperature and water supply and so highly valued that it is still common near the limits of its ecological zone. The annual sunlight requirement is above 2000 hours, with a likely lower limit of 120 hours per month. The optimum mean annual temperature is estimated at 27°C with average diurnal variation of 5–7°C. For good yields, a minimum monthly mean temperature of 20°C is required. Temperatures below 7°C may seriously damage young palms, but cultivars differ in their tolerance of low temperature. While most coconut palm is planted in areas below 500 m altitude, it may thrive at altitudes up to 1000 m, although low temperatures will affect growth and yield. Generally, coconut palm grows in areas with evenly distributed annual rainfall of 1000–2000 mm and high relative humidity, but it can still survive in drier regions if there is adequate soil moisture. The semi-xerophytic leaves enable coconut palm to minimize water loss and withstand drought for several months. Coconut palm thrives in a wide range of soils, from coarse sand to clay, provided they have adequate drainage and aeration. Coconut palm is halophytic and tolerates salt in the soil well. Coconut palm can grow in soils with a wide range of pH, but grows best at pH 5.5–7. | |||
| show more data (5) | comments (0) |
| Coconut palm is propagated by seed which is recalcitrant. The multiplication factor is low, as one palm will in general not produce more than 100–150 seed-nuts per year. Although plants can be regenerated through somatic embryogenesis, genotypic differences in rate of embryo formation and difficulties in hardening of in-vitro plants have been a constraint to practical methods of large-scale clonal propagation so far. In-vitro culture of excised embryos is also possible. It solves problems of plant quarantine restrictions and finds application in the international exchange of germplasm. Seed-nuts are usually given a resting period of one month after harvesting. They are kept in a germination bed from where uniform seedlings can be transplanted to polythene bags or to nursery beds. The polybag method and regular fertilization have largely replaced the bare-root seedlings raised in beds. Seedlings that are 5–8 months old are transplanted in the field. They can be kept longer in the nursery bed, but will then sustain a greater transplanting shock. Coconut palm is planted mostly at spacings of 8–10 m × 8–10 m, in a triangular or square system. Dwarf cultivars are planted at a spacing of 6–7 m × 6–7 m. Hedge planting may be used to facilitate intercropping, but the radial symmetry of the leaf arrangement does not tolerate extreme forms of row cropping. Many growers prefer wider spacing to prevent inter-tree competition. As the open crowns also transmit a fair portion of incident light coconut palm is well suited to intercropping. It is occasionally grown with cocoa and coffee. Although this usually results in lower copra yields, the combined income from well-fertilized coconut palm and intercrop is much higher than that from coconut palm alone. Coconut palm is also grown in mixed cropping systems with other crops such as rubber, mango, cashew, citrus and banana. Pastures are sometimes established under the palms for use in mixed husbandry. Green manures are also occasionally planted. However, pasture and cover crops can only be grown and maintained when there is sufficient rain. Catch crops such as rice, maize, finger millet, sweet potato, cassava, vegetables and spices are often planted until the palms come into bearing. These crops should not be planted closer than 2 m to the palms. | |||
| show more data (25) | comments (0) |
| Weeding is essential, especially for young coconut palms. Fertilizing is required, especially on soils that have been cultivated for many years, but smallholders seldom apply fertilizers due to limited financial resources. If nutrient deficiencies largely limit growth and yield, responses to organic and inorganic fertilizer application and other cultural practices such as cover crops and green manuring can be observed within one year. Potassium and chloride are the major nutrients needed by coconut palm, followed by nitrogen, phosphorus and sulphur. Leaf analysis is an accepted and quick guide to the fertilizer requirements of the palm. The annual crop nutrient removal of one ha of coconut palm, yielding 7000 nuts (1.0–1.3 t copra), is about 49 kg N, 16 kg P2O5, 115 kg K2O, 5 kg Ca, 8 kg Mg, 11 kg Na, 64 kg Cl and 4 kg S. Organic fertilizers have additional benefits of improving texture, water-holding capacity, cation exchange capacity and microflora of the soil, but generally cannot adequately compensate for crop nutrient removal, K in particular. An example of recommended inorganic fertilizer application per year and per palm is a mixture of 0.4 kg N, 0.3 kg P2O5, 1.2 kg K2O, 0.2 kg S and 0.9 kg Cl, applied in a band around the palm (l.0–1.5 m from the trunk) and split into 2 applications, at the beginning and end of the rainy season. Fertilizer doses depend on local conditions. Foliar and soil analyses help to determine the nutrient status of the palms. Irrigation is sometimes practised in dry areas where water is available and sea water may be applied occasionally as long as the salt content in the soil does not rise too high. | |||
| show more data (3) | comments (0) |
| Many diseases affect coconut palm. Serious threats to global coconut production are the lethal yellowing disease in the Caribbean and lethal yellowing-like diseases, such as the Kalimantan and Natuna wilts and Sulawesi yellows (Indonesia), Malaysian wilt, Socorro wilt (Philippines), Tatipaka disease and root wilt (India), leaf scorch decline (Sri Lanka), Awka disease (Nigeria), Cape St. Paul wilt (Ghana), Kaincopé disease (Togo), Kribi disease (Cameroon) and lethal disease (Kenya, Tanzania and Mozambique). The causal agent in each case is a related but distinct phytoplasma, as confirmed by molecular diagnostic assays. Generally, the symptoms of yellowing diseases are browning and collapse of spear leaves (leaves of full length, but still folded), yellowing of mature leaves, collapse of roots, premature nut fall, death of bud and later, of the tree. The probable vector of lethal yellowing disease is a plant hopper (Myndus crudus), but in all other cases implicated insect vectors have not yet been confirmed unambiguously. Blast disease and dry bud rot in coconut palm nurseries in Tanzania are probably also caused by a phytoplasma. Control measures include eradication of affected palms, plant quarantine and host resistance. Tall palms are generally susceptible. ‘Malayan Dwarf’ is highly tolerant of lethal yellowing disease, while ‘Pemba Red Dwarf’ shows resistance to lethal disease of East Africa. Kerala wilt, possibly caused by a virus, is an important disease in India. Cadang-cadang, caused by the cadang-cadang viroid (CCVD) is a devastating disease especially of flowering palms in the Philippines. Coconut palm in Guam is infected by a disease similar to cadang-cadang, also caused by a viroid. Bud rot occurs worldwide and is caused by the soil-borne fungus Phytophthora palmivora that is favoured by high humidity. It causes rotting of the spear leaf and growing point. It can be controlled by wider plant spacing, better aeration, drainage and weed control. Basal stem rot develops from an infection by the fungus Ganoderma boninense. The fungus first affects and destroys the roots and then the base of the stem turns reddish brown and releases a brown, gummy exudate. Disease occurrence can be prevented through improved growing conditions, production techniques and proper sanitation measures. Control methods are eradication of affected palms and application of fungicide. Stem bleeding or oozing of reddish brown liquid from the cracked stem is caused by Ceratocystis paradoxa (Thielaviopsis paradoxa). Cultural management techniques and drenching the soil with fungicides effectively control the disease. Leaf blight caused by Pestalotia palmarum and leaf rot or leaf spot caused by Drechslera halodes (Drechslera incurvata) are widespread fungal diseases. Leaf spot diseases caused by Cercospora spp. and Helminthosporium spp. in nurseries and young plantings in East Africa can be controlled by copper fungicides or mancozeb (e.g. Dithane M45). Numerous insect pests attack coconut palm. Several species of rhinoceros beetle are pests of coconut; the dominant species in Africa is Oryctes monoceros. Its larvae tunnel through the apical bud leaving characteristic triangular cuts in opened leaves. When the growing point is attacked, the palm dies. Control measures include removal of beetles from feeding tunnels, keeping the plantation free from dead stems, which are breeding grounds for the beetle, and trapping with an aggregation pheromone to reduce beetle populations. Other Coleoptera that inflict serious damage to coconut palm are Promecotheca spp., Brontispa longissima and Rhynchophorus spp. in Asia and the Pacific. Many caterpillars feed on the leaves, such as Hidari irava, Tirathaba spp., Setoria nitens, Parasa lepida and Artona catoxantha (Brachartona catoxantha) in Asia and Latoia pallida and Latoia viridissima in West Africa. Bacillus thuringiensis formulations can provide effective control in some cases. Coreid bugs (Pseudotheraptus wayi in East Africa and Pseudotheraptus devastans in equatorial Africa) attack flowers and young fruits, causing premature nut fall or deformed coconuts. Weaver ants (Oecophylla longinoda in Africa and Oecophylla smaragdina in Asia and the Pacific) are the most important natural enemy and stimulating their colonization of palms provides an effective method of biological control of this pest. Damage by termites (Macrotermes bellicosus in West Africa, Allodontermes morogorensis in East Africa) of seedlings in the nursery or newly planted fields should be prevented by timely nest destruction or chemical control with endosulfan or carbosulfan. | |||
| show more data (2) | comments (0) |
| Fruits of coconut palm can be harvested 11–12 months after flowering. The palm can be harvested every 2–3 months but rapidly germinating types should be harvested more frequently. Dwarf cultivars sprout in 45–60 days and must be harvested monthly. Climbing the palms and cutting the ripe bunches is still the harvesting method most practised. Gathering fallen coconuts is easier, but there are more losses due to rat attack and theft. Some coconuts may germinate on the tree and consequently their kernel and oil content may have started to deteriorate. In some countries bamboo poles (up to 25 m long) with a knife attached to the top end are used to cut the ripe bunches, elsewhere monkeys (Macacus nemestrina) are trained to harvest ripe nuts. | |||
| show more data (3) | comments (0) |
| Smallholder plantations usually yield between 0.5–1 t of copra/ha (30–50 fruits/palm). Well managed plantations of selected local tall coconut palm may yield 3–4 t copra/ha (90–130 fruits/palm). Plantations of dwarf coconut palm in Malaysia produce about 1.5–2 t copra/ha and even 3.5 t copra/ha under favourable conditions. Dwarf × tall hybrids combine the high number of fruits produced by the dwarf type with the larger fruit size from the tall one and usually have a higher yielding potential than the parents. Experimental yields of more than 6 t/ha of copra have been obtained in Côte d’Ivoire and the Philippines. | |||
| show more data (1) | comments (0) |
| Harvested coconuts are stored in a protected place until the husks are completely dry. Dried coconuts are husked manually by striking and twisting them on a steel point that is placed firmly in the ground. Husking machines have been developed but have not been a success. After husking, nuts are split with a machete and the water is drained. The nut halves are placed in a kiln dryer or an indirect hot air dryer for 1–2 days, after which the endosperm is scooped out from the shell and dried further until its moisture content is less than 6%. Sun drying is also practised but there is a higher risk of product deterioration especially during humid and rainy periods. Aflatoxin-producing moulds may affect the quality when the moisture content of dried copra exceeds 12%. Coconut oil can be extracted from the copra (yield about 60%) by dry processing methods such as mechanical pressing and by using solvents. It can also be extracted from the fresh kernel through several wet processes. The crude oil is subsequently cleaned by filtration, refined (chemically or by steam) to reduce its free fatty acid content, bleached (bleaching earth) to remove pigments and finally deodorized (stripping by steam) to produce a colourless cooking oil. The press cake, which still contains 6–10% oil, is ground to a meal and also pelletized if exported. In traditional extraction, coconut cream obtained from grated fresh kernel is boiled gently until the oil floats to the surface. Whole or husked coconuts are also sold to coconut desiccation factories. To produce desiccated coconut, the shell and the brown testa are pared off, the white endosperm is washed, steamed, pasteurized, shredded into small pieces of various sizes and forms, dried and packed. | |||
| show more data (1) | comments (0) |
| Local coconut palm cultivars (ecotypes) are usually heterogeneous populations with some predominating characteristics. Cultivars with different names and growing in different areas are sometimes rather similar and maybe of the same origin. Germplasm collections are maintained in several research stations around the world. In 1978 the International Board for Plant Genetic Resources (IBPGR, now IPGRI) adopted a minimum list of descriptors to be used in collecting germplasm in the field. In 1980 it supported the survey and collection of coconut germplasm in priority areas in South-East Asia and provided funds for the collection of coconut palms in Indonesia, the establishment of a coconut germplasm centre in the Philippines and collection of germplasm in the Pacific to be planted on one of the Andaman Islands to screen for Kerala wilt disease resistance for mainland India. The Coconut Genetic Resources Network (COGENT), with IPGRI’s administrative support, coordinates the conservation of more than 700 accessions in 15 countries. Major coconut germplasm collections include those of the Philippine Coconut Authority (PCA), the Research and Development Centre for Industrial Crops (RDCIC) in Indonesia, IPGRI-Asia, the Pacific and Oceania at Serdang, Malaysia, the Central Plantation Crop Institute (CPCRI) in India, the Instituto Nacional de Investigaciones Agrícolas, Irapa (INIA) in Venezuela, the National Centre for Agricultural Research (CNRA) in Côte d’Ivoire and the National Coconut Development Programme (NCDP) in Tanzania. Germplasm conservation by field collections requires considerable resources of land, staff and upkeep and remains vulnerable to natural disasters and diseases. The cryopreservation of embryos and pollen will enable the safe and inexpensive long-term storage of genetic resources. | |||
| show more data (3) | comments (0) |
| Breeding methods common to cross-pollinating species are applied to coconut palm. The long duration of one breeding generation (more than 10 years), low multiplication rate (1 : 50/100), recalcitrant and large seed and the large areas of land required for field testing, are major obstacles to rapid selection progress. About 95% of all planted coconut palm in the world are open-pollinated progenies after mass selection within local ecotypes, often informally applied by the growers themselves. Important selection criteria in coconut palm are: yield of copra and its components (number of fruits, copra content per fruit), early production, disease resistance and drought tolerance. Selection for endosperm thickness is a minor factor of selection, whereas oil content and quality are fairly constant. Length of husk fibres is a selection criterion in Sri Lanka only. The flavour of immature coconut water varies with ecotype, but has not been a criterion for formal selection as yet. The genetic variance in yield and its components is mainly due to additive genetic effects and the superior hybrids are the result of the general combining ability of the parents. Methods of (reciprocal) recurrent selection with genetically diverse sub-populations (dwarf and tall types) are now used in some breeding programmes to increase substantial transgressive hybrid vigour for yield in new cultivars. Molecular (e.g. microsatellite) markers have been recently developed in coconut palm to accurately assess genetic relationships between sub-populations. Dwarf × tall hybrids have considerable heterosis for yield and precocity; hence the focus of breeding programmes of several coconut research centres on such hybrids since 1960. Some 400 hybrids have been tested worldwide during the last 35 years; about 10 of these internationally at several locations. The coconut research centre at Port Bouet in Côte d’Ivoire tested 123 hybrids, of which 35 produced 65% more than the ‘West African Tall’ standard cultivar. Four hybrids yielded even more than twice as much (3.4–4.5 t/ha copra), including ‘PB121’ (‘Malayan Yellow Dwarf’ × ‘West African Tall’) which has been planted widely also in South-East Asia. Host resistance to major diseases has high priority in some areas, but sources of resistance are not always available, e.g. against Cadang-cadang disease in the Philippines. Crosses for breeding purposes are made by hand pollination after emasculation and bagging of inflorescences. Pollen collected from the male parent can be stored (dry and under vacuum) for a considerable period. Large-scale seed production is based on pollination of previously emasculated inflorescences (not bagged) in isolated seed gardens planted solely with the female parent of the hybrid cultivar (usually a dwarf type). One hectare of seed garden produces enough seed yearly for planting 50–60 ha. Hybrid seed production is rather expensive and requires large land areas. An estimated 15% of all coconut palms planted during the last decade are hybrids. Examples of widely planted hybrid cultivars are: the ‘KB’ and ‘KHINA’ series in Indonesia; the ‘PCA 15’ series in the Philippines and ‘PB’ series (e.g. ‘PB121’) from Côte d’Ivoire. | |||
| show more data (1) | comments (0) |
| Some of the latest dwarf × tall hybrid cultivars of coconut palm can potentially yield more than 6 t/ha of copra per year (3.7 t of oil), but coconut palm does not appear to have a bright future as a plantation crop in the long term. Coconut oil already faces increasing competition in the world market from palm-kernel oil and both may eventually also be partly replaced by lauric oils produced by genetically modified soya bean and brassica oilseed. On the other hand, as a smallholder crop in the coastal areas of the tropics, coconut palm will continue to be a very important supplier of multifunctional food and other products. Sometimes, it is practically the only crop that can be grown in the prevailing ecosystem (e.g. some Pacific Islands). A quickly growing world market for healthy and environmentally friendly products should offer new opportunities for the export trade. However, this will require astute marketing, more research into the economic viability of smallholder production systems (e.g. replanting, intercropping and biological control of diseases and pests) and into novel processing technologies for local industries to manufacture diversified products of coconut palm suitable for the international market. | |||
| show more data (2) | comments (0) |
| • Batugal, P.A. & Rao, V.R. (Editors), 1994. Coconut breeding. Workshop on Standardization of Coconut Breeding Research Techniques, 20–25 June 1994, Port Bouet, Côte d’Ivoire. International Plant Genetics Resources Institute, Regional Office for Asia, the Pacific and Oceania, Serdang, Malaysia. 150 pp. • Bourdeix, R., Baudouin, L., Billotte, N., Labouise, J.P. & Noiret, J.M., 1997. Le Cocotier. In: Charrier, A., Jackot, M., Hamon, S. & Nicolas, D. (Editors). L’Amélioration des plantes tropicales. CIRAD & ORSTOM, Montpellier, France. pp. 217–239. • Haas, A. & Wilson, L. (Editors), 1985. Coconut wood: processing and use. FAO, Rome, Italy. 58 pp. • Harries, H.C., 1995. Coconut (Cocos nucifera). In: Smartt, J. & Simmonds, N.W. (Editors): Evolution of crop plants. Longman, Scientific & Technical, Harlow, United Kingdom. pp. 389–394. • Harrison, N. & Jones, Ph., 2003. Diseases of coconut. In: Ploetz, R.C. (Editor). Diseases of tropical fruit crops. CABI Publishing, Wallingford, United Kingdom. pp. 197–225. • Lebrun, P., N’Cho, Y-P., Bourdeix, R. & Baudouin, L., 2003. Coconut. In: Hamon, P., Seguin, M., Perrier, X. & Glaszmann, J.Ch. (Editors). Genetic diversity of cultivated tropical plants. Science Publishers, Plymouth, United Kingdom & CIRAD, Montpellier, France. pp. 219–238. • Ohler, J.G. (Editor), 1999. Modern coconut management, palm cultivation and products. Intermediate Technology Publications, London, United Kingdom. 458 pp. • Ohler, J.G. & Magat, S.S., 2001. Cocos nucifera L. In: van der Vossen, H.A.M. & Umali, B.E. (Editors). Plant Resources of South-East Asia No 14. Vegetable oils and fats. Backhuys Publishers, Leiden, Netherlands. pp. 76–84. • Perera, L., Russell, J.R., Provan, J. & Powell, W., 2003. Studying genetic relationships among coconut varieties/populations using microsatellite markers. Euphytica 132: 121–128. • Rethinam, P., 2004. World coconut industry: past, present and future. Indian Coconut Journal 35(3): 3–14. • Schuiling, M., Mpunami, A., Kaiza, D.A. & Harries, H.C., 1992. Lethal disease of coconut palm in Tanzania 3: low resistance of imported germplasm. Oléagineux 47: 693–697. | |||
| show more data (70) | comments (0) |
| • Adkins, S., Samosir, Y., Nikmatullah, A., Wilkins R., Hetherington, S. & Ogle, H., 2002. Towards the clonal propagation of coconut. Acta Horticulturae 575: 107–115. • Arancon Jr, R.N., 1997. Asia-Pacific Forestry Sector Outlook Study: Focus on Coconut Wood. [Internet] FAO, Rome, Italy. http://www.fao.org/ documents/show_cdr.asp?url_file=/DOCREP/W7731E/ w7731e07.htm. Accessed May 2006. • Child, R., 1974. Coconuts. 2nd edition. Longmans, London, United Kingdom. 335 pp. • Menon, K.P.V. & Pandalai, K.M., 1958. The coconut palm, a monograph. Indian Central Coconut Committee, Ernakulam, India. 384 pp. • Mwinjaka, S., Chiduza, C., Temu, A.E., Sukume, C. & Diehl, L., 2000. Coconut palm replacement model for Tanzanian farming systems. Journal of Agricultural Economics and Development 3: 61–70. • Oehlschlager, C.A.M., 2004. Current status of trapping palm weevils and beetles. Planter 81: 123–143. • Perry, L.M., 1980. Medicinal plants of East and Southeast Asia: attributed properties and uses. The MIT Press, Cambridge, Massachusetts, United States and London, United Kingdom. 620 pp. • Tsai, J.H. & Harrison, N.A., 2003. Lethal yellowing of coconut and lethal decline of palms. In: Loebenstein, G. & Thottappily, G. (Editors). Virus and visus-like diseases of major crops in developing countries, Kluwer Academic Publishers, Dordrecht, the Netherlands. pp. 597–606. | |||
| show more data (70) | comments (0) |
| show more data (20) | comments (0) |
| • Ohler, J.G. & Magat, S.S., 2001. Cocos nucifera L. In: van der Vossen, H.A.M. & Umali, B.E. (Editors). Plant Resources of South-East Asia No 14. Vegetable oils and fats. Backhuys Publishers, Leiden, Netherlands. pp. 76–84. | |||
| show more data (0) | comments (0) |
| |||||||
| |||||||
| |||||||
| |||||
| van der Vossen, H.A.M. & Chipungahelo, G.S.E., 2007. Cocos nucifera L. [Internet] Record from PROTA4U. van der Vossen, H.A.M. & Mkamilo, G.S. (Editors). PROTA (Plant Resources of Tropical Africa / Ressources végétales de l’Afrique tropicale), Wageningen, Netherlands. <http://www.prota4u.org/search.asp>. Accessed . | |||
| There are 23 study abstracts related to Cocos nucifera L.. Click on "show more" to view them. | ||
| show more data | comments (0) |
| There are 100 book citations related to Cocos nucifera L.. Click on "show more" to view them. | ||
| show more data | comments (0) |
| There are 101 citation in web searches related to Cocos nucifera L.. Click on "show more" to view them. | ||
| show more data | comments (0) |
| There are 89 citation in scholarly articles related to Cocos nucifera L.. Click on "show more" to view them. | ||
| show more data | comments (0) |
| There are 100 news article citations related to Cocos nucifera L.. Click on "show more" to view them. | ||
| show more data | comments (0) |
| There are 20 citations in Afrirefs related to Cocos nucifera L.. Click on "show more" to view them. | ||
| show more data | comments (0) |
| There are 39 Wikipedia citations related to Cocos nucifera L.. Click on "show more" to view them. | ||
| show more data | comments (0) |
| General importance |  |
| Geographic coverage Africa |  |
| Geographic coverage World |  |
| Vegetables |  |
| Ornamental use |  |
| Forage/feed use |  |
| Timber use |  |
| Carbohydrate/starch use |  |
| Fuel use |  |
| Medicinal use |  |
| Vegetable oil use |  |
| Fibre use |  |
| Climate change |  |
| Food security |  |