Record display
 PROTA4U Homepage
PROTA4U Homepage Select translation pop-up: Select translation pop-up: |
 |
PROTA4U Record display |
 PROTA4U Homepage PROTA4U Homepage
|
| Sp. pl. 2: 741 (1753). | |||
| show more data (14) | comments (0) |
| Papilionaceae (Leguminosae - Papilionoideae, Fabaceae) | |||
| show more data (16) | comments (0) |
| 2n = 40 | |||
| show more data (19) | comments (0) |
| show more data (15) | comments (0) |
| Groundnut, peanut, earthnut, monkey nut (En). Arachide, cacahuète, cacahouète, pistache de terre (Fr). Amendoim, mandobi, caranga (Po). Mjugu nyasa, mnjugu nyasa, karanga (Sw). | |||
| show more data (47) | comments (0) |
| Groundnut originated in the area of southern Bolivia and north-western Argentina. It is an ancient crop of the New World and was widely grown in Mexico, Central America and South America in pre-Columbian times. Domesticated groundnut had already evolved into several types before it was introduced into the Old World by Spanish and Portuguese explorers. Two-seeded types originating from Brazil were brought to West Africa, and 3-seeded types originating from Peru were taken from the west coast of South America to the Philippines, from where they spread to Japan, China, Indonesia, Malaysia, India, Madagascar and East Africa. In the late 1700s ‘Spanish’ groundnut types were introduced into Europe from Brazil. The first successful introduction in North America concerned small-seeded ‘runner’-type groundnuts, probably originating from northern Brazil or the West Indies. Groundnut is now grown in most tropical, subtropical and temperate countries between 40°N and 40°S latitude. It is grown throughout tropical Africa and is a major cash crop in Senegal, Gambia, Nigeria and Sudan. | |||
| show more data (41) | comments (0) |
| Groundnut seed is mainly used as food and for oil extraction. The seeds are eaten raw, boiled or roasted, made into peanut butter, confectioneries and snack foods, and are used for thickening soups or made into sauces to be eaten with meat and rice. In northern Nigeria groundnut flour is mixed with ‘gari’ (coarse fermented cassava meal) and made into balls that are eaten as a snack. In the United States and Argentina most of the crop is used as food, but in most other countries the primary use of groundnut is for the oil market. Worldwide, more than 50% of groundnut production is crushed into oil for human consumption or industrial use (e.g. in cosmetics). In countries such as Senegal, Gambia and Nigeria oil extraction has been an important cottage industry for years. The use of groundnut in confectionery and for oil and meal production is increasing, and there is gradual shift taking place from oil and meal to confectionery use, especially in Latin America and the Caribbean. In South America groundnut seeds are fermented into alcoholic drinks. The presscake from oil extraction is a feed rich in protein, but it is also made into groundnut flour, which is used in many human foods. Fermented groundnut cake is eaten fried in Indonesia. The cake finds industrial application in the production of glues, sizes for paper and starches for laundering and textile manufacture. Protein from groundnut cake is made into a wool-like fibre, which can be blended with wool or rayon. Groundnut shells are used as roughage in fodder, as fuel, fertilizer, mulch, in the manufacture of particle board and building blocks, and can be used as a source of activated carbon, combustible gases, organic chemicals, reducing sugars, alcohol and extender resins. Young groundnut pods and leaves are consumed as a vegetable; in West Africa the leaves are added to soups. The foliage is an important fodder, especially in the Sahel; it may be eaten fresh or as hay or silage. In southern India the haulms are sometimes applied as a green manure. Groundnut has a range of uses in traditional African medicine. Pod extracts are taken as a galactagogue, and used as eye-drops to treat conjunctivis. Macerations of peeled seeds are drunk to treat gonorrhoea, macerations of the seed coats against syphilis, while macerations of the seed coats and shells are applied against ophthalmia. Sap of ground leaves and seeds is used for ear-drops against ear discharge. Leaf macerations are drunk as a diuretic. Leaf infusions are drunk against female infertility, and used for eye-drops to treat eye injuries and cataract. Plant ash with salt is applied in case of caries. Pod extracts and young plants are credited with aphrodisiac properties. The plant is also used to relieve cough and is considered emollient and demulcent; emulsions are taken to treat pleurisy, enteritis (including colitis), and dysuria. Agglutinins (lectins) from groundnut seeds are often used in medical research for histochemical investigations. | |||
| show more data (25) | comments (0) |
| According to FAO estimates, the average world production of groundnut pods in 1999–2003 amounted to about 34.4 million t/year from 24.4 million ha. The main producing countries are China (14.0 million t/year in 1999–2003, from 4.9 million ha), India (6.1 million t/year from 6.7 million ha), Nigeria (2.8 million t/year from 2.7 million ha), the United States (1.7 million t/year from 0.5 million ha), Indonesia (1.3 million t/year from 0.7 million ha) and Sudan (1.1 million t/year from 1.7 million ha). The total production in sub-Saharan Africa was 8.2 million t/year from 9.5 million ha. Average world export of groundnut seeds amounted to 1.1 million t/year in 1998–2002. The main exporters were China (321,000 t/year), Argentina (201,000 t/year) and the United States (171,000 t/year). Export of groundnut seeds from sub-Saharan Africa was 64,000 t/year, with Gambia as main exporter (26,000 t/year). Average world export of groundnut pods in 1998–2002 was only 176,000 t/year, with China as main exporter (73,000 t/year). Exports of groundnut pods from sub-Saharan Africa were negligible. The world production of groundnut oil in 1999–2003 was 5.1 million t/year. The main producers are China (2.0 million t/year), India (1.4 million t/year), Nigeria (480,000 t/year), Senegal (178,000 t/year) and Sudan (162,000 t/year). The production in sub-Saharan Africa was 1.2 million t/year. The world groundnut cake production in 1999–2003 was 6.9 million t/year, mainly from China (2.6 million t/year), India (1.9 million t/year) and Nigeria (750,000 t/year). The production in sub-Saharan Africa was 1.6 million t/year. Average world export of groundnut oil in 1998–2002 was 271,000 t/year, with as main exporters Senegal (83,000 t/year) and Argentina (69,000 t/year). The total export of groundnut oil from sub-Saharan Africa was 114,000 t/year. The main importers were France (68,000 t/year), Italy (46,000 t/year) and the United States (25,000 t/year). Average groundnut cake export amounted to 280,000 t/year. Major exporters were Senegal (103,000 t/year), Argentina (51,000 t/year), India (43,000 t/year) and Sudan (35,000 t/year). Total groundnut cake export from sub-Saharan Africa was 143,000 t/year. The main importers were France (129,000 t/year) and Thailand (53,000 t/year). | |||
| show more data (3) | comments (0) |
| Mature groundnut seeds contain per 100 g edible portion (average of several types, which show little difference): water 6.5 g, energy 2374 kJ (567 kcal), protein 25.8 g, fat 49.2 g, carbohydrate 16.1 g, dietary fibre 8.5 g, Ca 92 mg, Mg 168 mg, P 376 mg, Fe 4.6 mg, Zn 3.3 mg, vitamin A 0 IU, thiamin 0.64 mg, riboflavin 0.14 mg, niacin 12.1 mg, vitamin B6 0.35 mg, folate 240 μg and ascorbic acid 0 mg. The essential amino-acid composition per 100 g edible portion is: tryptophan 250 mg, lysine 926 mg, methionine 317 mg, phenylalanine 1337 mg, threonine 883 mg, valine 1082 mg, leucine 1672 mg and isoleucine 907 mg. The principal fatty acids are per 100 g edible portion: oleic acid 23.7 g, linoleic acid 15.6 g and palmitic acid 5.2 g (USDA, 2004). Groundnut seeds yield 42–56% oil. Groundnut oil contains 36–72% oleic acid, 13–48% linoleic acid and 6–20% palmitic acid. The ratio of oleic to linoleic acid has an important bearing on the stability of the oil; the higher the ratio, the more stable the oil and the longer its shelf life. The ratio in mature seeds can range from less than 1.0 to greater than 3.0; more than 1.3 is generally considered satisfactory by processors. The presscake contains 40–50% easily digestible protein, 20–25% carbohydrate and 5–15% residual oil. Groundnut pods have a thick woody shell containing normally 2–3 seeds (‘kernels’). The seed coat constitutes about 4–5% of the seed weight, the cotyledons 90–94% and the germ 3–4%. The major components of the seed coat are carbohydrate, cellulose and protein. Oil and protein are the main constituents of the germ and cotyledons. The germ is associated with bitter components. An important problem in groundnut production is aflatoxin contamination by Aspergillus fungi. Aflatoxin has immunosuppressive effects and epidemiological studies, also in Africa, have shown a positive correlation between aflatoxin intake and the incidence of liver cancer. After industrial oil extraction, aflatoxin remains in the cake, and the refined oil is free of aflatoxin, but in case of small-scale extraction, the non-refined oil may be contaminated. Groundnut is one of the most allergenic foods known and may cause anaphylactic reactions. Groundnut seeds contain a haemostatic factor which can be useful in haemophilia. Groundnut oil is mildly laxative. | |||
| show more data (4) | comments (0) |
| Groundnut oil can be substituted by other vegetable oils, e.g. from maize, soya bean and sunflower. | |||
| show more data (0) | comments (0) |
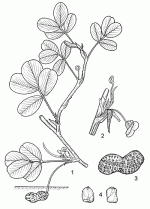
| Annual herb, with erect or prostrate stem up to 70 cm long; root system consisting of a well-developed taproot with many lateral roots, up to 135 cm deep, but generally restricted to the upper layers of the soil. Leaves arranged spirally, 4-foliolate with two opposite pairs of leaflets; stipules 1.5–4 cm long, with a slender free tip, but fused to the petiole for about half their length; petiole 1.5–7 cm long; petiolules 1–2 mm long; leaflets obovate or elliptical, 1–7 cm × 0.5–3 cm, cuneate-rounded at base, rounded or emarginate and mucronate at apex. Inflorescence an axillary, 2–5-flowered spike. Flowers bisexual, papilionaceous, sessile; receptacle long and slender, pedicel-like, up to 4 cm long; calyx with 4 upper lobes joined, lower lobe free; corolla pale yellow to orange-red, rarely white, standard rounded, c. 1.5 cm × 1.5 cm, wings shorter, keel incurved; stamens (8–)10, alternately with small, globular anthers and larger, oblong anthers, joined at base; ovary superior but situated at base of receptacle tube, style free within the tube, very long, ending in a minute club-shaped stigma. Fruit an oblong or sausage-shaped pod, borne at the tip of an elongated fruit stalk (‘peg’) up to 20 cm long, 1–8 cm × 0.5–2 cm, surface constricted to varying degrees between the seeds and reticulately veined, 1– 6-seeded. Seeds cylindrical to ovoid, 1–2 cm × 0.5–1 cm, with pointed or flattened ends, enclosed in a thin papery seed coat ranging in colour from white to deep purple. Seedling with epigeal germination; cotyledons thick and fleshy. | |||
| show more data (14) | comments (0) |
| Arachis comprises about 70 species, all distributed in South America. The centre of origin of Arachis is the Mato Grosso region of Brazil. Arachis hypogaea is by far the most economically important species in this genus, but several other species have been cultivated for their seeds, including Arachis villosulicarpa Hoehne and Arachis stenosperma Krapov. & W.C.Greg. High levels of resistance to many diseases and pests of groundnut have been recorded in other Arachis species. Many of them are closely related to groundnut and include the other 26 species in section Arachis. Several diploid species have been suggested as wild progenitors of groundnut, but molecular and cytogenetic studies indicate that Arachis duranensis Krapov. & W.C.Greg. and Arachis ipaensis Krapov. & W.C.Greg. are most closely related to the progenitors of allotetraploid domesticated groundnut. Arachis monticola Krapov. & Rigoni is the only other tetraploid species in the section; it is very closely related to Arachis hypogaea and may be the direct descendant of the original hybrid between the 2 diploid progenitor species. Hybrids between Arachis hypogaea and other Arachis species have been produced by direct hybridization and by first creating autotetraploids or allotetraploids from the diploid species before making crosses. Hybrids show high levels of sterility due to ploidy level differences and genome incompatibility. There is considerable variation in Arachis hypogaea and two subspecies have been distinguished: subsp. hypogaea and subsp. fastigiata Waldron. Subsp. hypogaea (‘runner type’) is characterized by a more prostrate growth habit without flowering branches on the main stem, and with the cotyledonary lateral branches carrying alternate pairs of vegetative and reproductive secondary branches; it is usually late-maturing. It includes the ‘Virginia’ types groundnut. Subsp. fastigiata (‘bunch type’) is characterized by an erect growth habit with flowering branches on the main stem, and without a regular pattern in the sequence of vegetative and reproductive branches; and it is early-maturing. It includes the ‘Spanish’ and ‘Valencia’ types groundnut. Most groundnut cultivars grown in West Africa belong to subsp. hypogaea; most of those in East Africa to subsp. fastigiata. Subsp. hypogaea is mainly used for food, and subsp. fastigiata, which has a higher oil content, as a source of oil. | |||
| show more data (27) | comments (0) |
| Seeds of ‘Virginia’ types have a dormancy period of 1–3 months, whereas ‘Spanish’ and ‘Valencia’ types are without dormancy. The optimum soil temperature for seed germination is 25–30°C. Low temperatures retard germination and development and increase the risk of seedling diseases. Upon germination, the primary root elongates rapidly, reaching 10–12 cm before lateral roots appear. As growth proceeds, the outer layer of the primary root of a seedling is sloughed off so that root hairs do not form. Branching is dimorphic, with vegetative branches and reduced reproductive branches. Secondary and tertiary vegetative branches can develop from the primary vegetative branches. Flowering may start as early as 20 days after planting, but 30–40 days after planting is more usual. The number of flowers produced per day decreases as the seeds mature. Up to 50% of the embryos may abort even under ideal environmental conditions, but this percentage becomes much higher during times of drought or other environmental stress. However, plants can produce a ‘second crop’ of seeds if adequate moisture becomes available again. Groundnut is self-pollinating, but outcrossing can occur when bees pollinate the flowers. Groundnut generally produces more flowers under long day conditions, but reproductive efficiency is greater under short days. Only one of the flowers in an inflorescence opens at a given time. Flowers wither within 24 hours after anthesis. Fertilization usually occurs within 6 hours after pollination, when the basal part of the ovary starts elongating into a structure called ‘peg’. The embryo initiates a growth phase until it reaches an 8–16-cell stage. It then becomes quiescent during the 5–15 days required for the ‘peg’ to enter the soil. The ‘peg’ stops elongating within a day or two after soil penetration, the embryo then restarting growth. In wild Arachis species the ‘peg’ may continue to grow to a length of nearly 2 m. Seeds in ‘Spanish’-type cultivars usually mature within 90–120 days after planting, whereas ‘Virginia’-type cultivars take 130 days or more. Pods of the same size may differ significantly in maturity and seed weight. Groundnut is usually effectively nodulated by N2-fixing Bradyrhizobium bacteria. Because root hairs are absent, the bacteria infect the root through cracks in the epidermis near multicellular hairs at the basis of the root. | |||
| show more data (5) | comments (0) |
| The optimum mean daily temperature for groundnut growth is 27–30°C; growth ceases when temperature drops below 15°C. Groundnut is mainly grown in areas with an average annual rainfall of 500–1000 mm; 500–600 mm of rain reasonably well distributed over the growing season allows satisfactory production. Nevertheless, groundnut is drought-tolerant and can withstand severe lack of water, though yield is generally reduced. A dry period is required for ripening and harvesting. The phenology of groundnut is determined primarily by temperature, with cool temperatures delaying flowering. In controlled environments, photoperiod has been shown to influence the proportion of flowers producing pods and distribution of assimilates between vegetative and reproductive structures (harvest index) in some cultivars. Long photoperiods (greater than 14 hours) generally increase vegetative growth and short photoperiods (less than 10 hours) increase reproductive growth. Groundnut can be grown up to 1500 m altitude. The best soils for groundnut are deep (at least 30–40 cm), friable, well-drained sandy loams, well-supplied with calcium and a moderate amount of organic matter. It is important to maintain near to neutral soil pH levels and Ca:K ratios lower than 3. | |||
| show more data (7) | comments (0) |
| Groundnut is propagated by seed, but vegetative propagation using cuttings is possible. The 1000-seed weight ranges from 150 g to more than 1300 g. Sowing high-quality seed in a well-prepared, moist seedbed is essential for crop establishment. Groundnut seeds are often planted at a depth of 4–7 cm at a rate of 60–80 kg/ha. Groundnut pods intended for sowing are often hand-shelled 1–2 weeks before sowing. Only fully mature pods are selected. Before sowing, groundnut seed may be treated with a fungicide to control seedling diseases. In general, early sowing improves yields and seed quality. Early sown crops also suffer less risk of disease such as groundnut rosette virus. However the appropriate sowing date depends on the maturity period of the cultivar. Small-seeded ‘Spanish’ types are spaced at 60–75 cm between rows and 10 cm within the row. This gives an optimum plant population of 133,000–167,000 plants per ha. Large-seeded ‘Virginia’ types are spaced at 75 cm between rows and 15 cm within the row, giving an optimum plant population of 89,000 plants per ha. Groundnut can be grown on the flat, or on ridges as is often the case in Malawi. Groundnut grown on ridges tends to give higher yields, probably because of more loose soil favourable for pod development and easier uprooting. In tropical Africa groundnut is grown as a sole crop or intercropped between rows of cereals such as maize, sorghum or pearl millet. | |||
| show more data (8) | comments (0) |
| Groundnut does not compete effectively with weeds, particularly in the early stages of development. The crop should be thoroughly weeded within the first 45 days. Once the development of the ‘peg’ begins, earthing-up is kept to a minimum. Weeds at this stage are hand pulled. Pre-and post-emergence herbicides may be used to eradicate weeds, but they are too expensive for most small-scale farmers in Africa. In sound rotation systems, groundnut benefits from residual fertility; in general no additional fertilizer is given if the crop is sown on a well-managed soil previously treated with a balanced fertilizer. However, in order to ensure good crop establishment, high yield and good seed quality, a fertilizer containing Ca, such as gypsum or single superphosphate, should be applied. Calcium is absorbed directly by the pods if soil moisture is adequate. A shortage of Ca in the zone where the pods develop will result in empty pods, particularly in cultivars of the ‘Virginia’ type. Groundnut is normally a rainfed crop, but it is grown under irrigation in Sudan. Groundnut should preferably not be grown in the same field more than once in 3 years to limit damage by soil-borne diseases, nematodes and weeds. It fits into a wide range of rotations and it can follow any clean-weeded crop, e.g. maize, sorghum, pearl millet, cassava, sweet potato or sunflower. To reduce the incidence of diseases and pests, groundnut should not be sown after cotton or tobacco. Groundnut does well on virgin land or immediately following a grass ley or well-fertilized crop such as maize. The intensity of management of groundnut varies considerably around the world, depending on the economic return for the crop or the role of groundnut in the farming system. In the United States, Australia and parts of South America the crop is grown with intensive management, generally with high levels of mechanical and chemical inputs. In many countries groundnut is grown as a cash crop primarily for export. | |||
| show more data (2) | comments (0) |
| Groundnut is susceptible to a number of diseases, such as early leaf spot (Cercospora arachidicola), late leaf spot (Cercosporidium personatum, synonym: Cercospora personata), rust (Puccinia arachidis), groundnut rosette (caused by a complex of 3 agents: groundnut rosette virus (GRV), groundnut rosette assistor virus (GRAV) and a satellite RNA) and aflatoxin contamination caused by Aspergillus fungi. Foliar diseases of groundnut are among the most important yield-limiting factors in groundnut production. Early and late leaf spots and rust together may cause up to 70% yield losses; even where fungicides are applied significant yield reductions occur. Spraying with fungicide when the disease appears controls both leaf spots effectively. Dusting groundnut leaves with sulphur, early in the morning when there is still dew on the leaves, has been reported to control both early and late leaf spots. The use of sulphur has also been observed to increase leaf retention, thus increasing the quantity of leafy stems available for livestock feed. Cultural practices to control leaf spots include crop rotation and burning of crop residues. Cultivars with partial resistance to leaf spots have been developed. Rust generally occurs sporadically and at low severity, although it can cause crop losses up to 40% when an epidemic occurs. The cultural practices and fungicidal control measures recommended for leaf spots are also applicable to rust. Resistant cultivars are available. Groundnut rosette virus, transmitted by the aphid Aphis craccivora, is endemic to sub-Saharan Africa and widely prevalent in Ghana, Nigeria, Malawi and Zambia. It is the most destructive disease of groundnut leading to 30–100% yield loss. Early sowing at high plant populations controls the spread of groundnut rosette by giving complete soil coverage as quickly as possible and restricting the movement of aphids. Cultivars resistant to groundnut rosette are widely grown in Africa. In Malawi it is common practice for farmers to interplant groundnut and cowpea to control groundnut rosette. Aspergillus fungi can invade groundnut pods and seeds, producing toxic compounds known as aflatoxin. Contaminated produce can be poisonous to people and livestock, and cannot be exported. Aflatoxin contamination also affects groundnut seed, leading to low germination percentage and poor seedling establishment. It can occur before harvest, during field drying and curing, and in storage. Pre-harvest contamination is likely to be most serious under drought. Post-harvest contamination occurs if groundnut pods or seeds become moist and/or damaged. Various methods are used to control aflatoxin. They include avoiding mechanical damage to pods or seeds during weeding, harvesting and storage, harvesting as soon as the pods are mature, proper drying and curing, and storing in the shell at low temperature under moisture-free conditions. Root-knot nematodes (Meloidogyne spp.) may cause considerable yield loss in groundnut; they can be controlled by crop rotation. On a global scale the most important insect pests include aphids (Aphis craccivora), thrips (Frankliniella spp.), jassids (Empoasca dolichi), white grubs (larvae of various beetles), termites (mainly Microtermes sp.) and the red tea bug Hilda patruelis. False wireworms and millipedes seem to occur less frequently. In general, soil pests cause more damage than foliage feeders or sucking pests. However, aphids are particularly harmful because they transmit groundnut rosette virus. In Asia and Africa white grubs, termites, millipedes and ants are important pests; in the United States the lesser cornstalk borer (Elasmopalpus lignosellus) and the southern corn rootworm (Diabrotica undecimpunctata) are the main insect pests of groundnut. Pests attacking stored groundnut pods and seeds include bruchids (Caryedon serratus, Callosobruchus spp., Acanthoscelides spp.) and flour beetles (Tribolium spp.). Parasitic plants (Alectra vogelii Benth. and Striga spp.) are recorded as causing damage to groundnut in various African countries. | |||
| show more data (3) | comments (0) |
| The indeterminate flowering pattern of groundnut makes proper timing of harvest difficult, even though such timing is crucial for obtaining maximum yield and quality. Harvest at the proper time ensures that the maximum number of pods have attained their greatest weight and that pods are not falling off. Methods to determine the proper time for harvesting groundnut are available, but some are environment-specific or are prohibitively expensive. Presently only the shell-out method and the hull-scrape method are widely used for groundnut maturity determination. The shell-out method is based on colour changes within the pod wall ( ‘hull’) that occur as the pod matures. The internal pod wall surface of most cultivars changes from white to brown or black blotches covering a large percentage of the area. The colour of the seed coat changes from white to dark pink or tan at the same time. A sample of plants is taken and pods opened. The percentage of pods with dark colour inside the pod wall is determined. Harvesting should begin when the percentage is 60–80, but recommendations vary. The shell-out method is widely used because it can directly be used in the field without further handling of pods, requires no equipment and provides an immediate answer. The hull-scrape method, developed in the early 1990s, is currently accepted as the most accurate means of assessing the maturity of ‘runner’-type groundnuts. The method is based on the fact that the pod mesocarp (the area just beneath the pale brown coloured exterior of the groundnut pod) changes from white to yellow to orange to brown to black as the crop matures. The method requires colour charts and a pocket knife to scrape the pod surface. Harvesting is carried out manually in most parts of Africa, as well as Asia. In the United States harvesting is normally done using a digger shaker inverter. When plants are harvested manually, they are loosened with a hoe and pulled out of the ground, after which they are turned to expose the pods to the sun to facilitate drying. When dry, the pods are ripped off the plants. With mechanical harvesting, the plants are cleanly removed from the soil and deposited in inverted windrows. Pods have to remain in the windrows until the average moisture content is 18–24%. Pods are then picked using a combine. Rainfall during windrowing may promote mould growth resulting in reduced milling quality. | |||
| show more data (3) | comments (0) |
| In tropical Africa the average yield of groundnut pods in the early 2000s was about 850 kg/ha, which is only slightly higher than the average yield in the 1970s (730 kg/ha). National average yields of groundnut pods in tropical Africa range from 300–1000 kg/ha. Average world yields of groundnut pods increased from 0.9 t/ha in the 1970s to 1.4 t/ha in the early 2000s. With good management practices and proper disease control, yields up to 5 t/ha can be achieved. On average 100 kg of pods yield 70 kg of seeds, containing 35 kg oil. | |||
| show more data (1) | comments (0) |
| Produce quality is closely related to proper harvesting date, harvesting method and drying; every step is critical to obtaining or maintaining quality. Groundnut pods are dried to an average moisture content of about 10%. Removing foreign materials early helps to maintain quality during storage. Cleaning equipment to remove the foreign material has been developed and includes sand screens and belt screens. Groundnut pods are stored in granaries, tanks, bins, concrete silos, warehouses or in the open. In storage, ventilation is crucial to prevent moisture build up which can promote mould growth and aflatoxin production. Excessive heat should be avoided. Storage structures should be examined frequently for moisture and insect problems as these can greatly reduce quality. Seeds can be protected from mechanical damage by storage and transport in the pods. In many areas groundnut is only shelled when it is to be used or sold; in local markets unshelled pods are often offered for sale. Both mechanical and manual shelling are common. Groundnut removed from storage is transported to shelling centres where the pods are graded, cleaned and shelled, and the seeds are separated into commercial grade sizes. Shelling operations may damage the seeds. Shelling of 100 kg of groundnut pods yields 60–80 kg of seeds. Generally groundnut seeds can be stored at 1–5°C and 50–70% relative humidity for 1 year without loss of quality. Groundnut seeds tend to absorb gases and off-flavours, which should be avoided. Oil is extracted from groundnut seed by expeller pressing, hydraulic pressing, solvent extraction, or a combination of these methods. Expeller pressing is most widely used. | |||
| show more data (1) | comments (0) |
| The International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, India, holds the largest collection of groundnut types, with more than 15,000 accessions, differing for many vegetative, reproductive, physiological and biochemical traits including their reactions to biotic and abiotic stresses. A duplicate sample is maintained in a regional gene bank at Niamey, Niger. Other large collections of groundnut germplasm are held in the United States (Southern Regional Plant Introduction Station, Griffin, Georgia, 9000 accessions), India (National Research Centre for Groundnut (NRCG), Junagadh, 8000 accessions), China (Institute of Crop Germplasm Resources (CAAS), Beijing, 5400 accessions; Institute of Oil Crops Research, Wuhan, 5700 accessions). In tropical Africa substantial groundnut germplasm collections are held in Senegal (Centre National de Recherche Agronomique, Bambey, 900 accessions), Uganda (Serere Agricultural and Animal Production Research Institute, Serere, 900 accessions) and Malawi (Plant Genetic Resources Centre, Chitedze Agricultural Research Station, Lilongwe, 500 accessions). The ARC Grain Crops Institute in Potchefstroom, South Africa, has a collection of 850 accessions. Core collections that have been developed are useful for developing models for future germplasm acquisition and evaluation for disease resistance. Additional collections are needed for most groundnut-producing regions, as landraces in these areas are rapidly being replaced with modern cultivars. | |||
| show more data (2) | comments (0) |
| Groundnut breeding efforts greatly increased when the ICRISAT groundnut breeding programme was established in 1976. Diverse breeding populations are now being tested in regional programmes in sub-Saharan Africa and Asia. Most breeding programmes are conducted by public institutions. Groundnut breeding objectives have concentrated on adaptation to regional markets and production systems. All programmes aim at improving the productivity of the crop and resistance to diseases. Large-scale efforts to evaluate wild Arachis germplasm have resulted in identification of useful sources of resistance to many diseases. Recently there have been initiatives to improve flavour and quality. Breeding for resistance to aflatoxin contamination has received increased attention, and the selection of short-duration cultivars with drought resistance is a high priority in many programmes. Commonly used breeding methods in groundnut are pedigree selection, bulk-pedigree selection and single-seed descent. Backcross breeding has not been used extensively as most of the economically important traits of groundnut are quantitatively inherited. The major constraints to rapid genetic enhancement include: the close linkage of disease resistance genes with loci conferring undesirable pod and seed characteristics; the later maturity, lower partitioning to seeds, and higher photoperiod-sensitivity of disease-resistant germplasm compared to agronomically elite susceptible materials; the large genotype × environment interactions for traits of economic importance; and limited gene introgression from wild Arachis species to cultivated groundnut. Genetic linkage maps of groundnut have been constructed using various markers, but the saturation level is insufficient for routine application in molecular breeding. An efficient tissue culture and transformation system for groundnut has been developed and transgenic groundnut plants have been produced using biolistic and Agrobacterium -mediated methods. | |||
| show more data (2) | comments (0) |
| Groundnut remains an extremely useful crop, providing food, oil, fodder and fuel to households and is also an important source of additional income as a cash crop. Important problems in groundnut cultivation in tropical Africa are low yields and its susceptibility to diseases. Many cultivars are still susceptible to early and late leaf spot and rust, as resistance tends to be linked with long duration and undesirable pod and seed characteristics. Therefore, the development of high-yielding cultivars with resistance to disease (especially leaf spots and rust) and adaptation to African production systems remains a major challenge for groundnut breeders. The application of DNA markers may allow breeders to combine resistance to biotic and abiotic stresses with improved productivity and seed quality. The use of biotechnology tools will become increasingly important for large-scale germplasm characterization and resolving some of the constraints (e.g. disease problems) in groundnut production. | |||
| show more data (3) | comments (0) |
| • Dwivedi, S.L., Crouch, J.H., Nigam, S.N., Ferguson, M.E. & Paterson, A.H., 2003. Molecular breeding of groundnut for enhanced productivity and food security in the semi-arid tropics: opportunities and challenges. Advances in Agronomy 80: 153–221. • Knauft, D.A. & Ozias-Akins, P., 1995. Recent methodologies for germplasm enhancement and breeding. In: Patte, H.E. & Stalker, H.T. (Editors). Advances in peanut science. American Peanut Research and Education Society, Stillwater, Oklahoma, United States. pp. 54–94. • Knauft, D.A. & Wynne, J.C., 1995. Peanut breeding and genetics. Advances in Agronomy 55: 393–445. • Kokalis-Burelle, N., Porter, D.M., Rodríguez-Kábana, R., Smith, D.H. & Subrahmanyam, P. (Editors), 1997. Compendium of peanut diseases. 2nd Edition. APS Press American Phytopathological Society, St. Paul, Minnesota, United States. 94 pp. • Krapovickas, A. & Gregory, W.C., 1994. Taxonomia del genero Arachis (Leguminosae). Bonplandia 8(1-4): 1–186. • Melouk, H.A. & Shokes, F.M. (Editors), 1995. Peanut health management. APS Press American Phytopathological Society, St. Paul, Minnesota, United States. 117 pp. • Shorter, R. & Patanothai, A., 1989. Arachis hypogaea L. In: van der Maesen, L.J.G. & Somaatmadja, S. (Editors). Plant Resources of South-East Asia No 1. Pulses. Pudoc, Wageningen, Netherlands. pp. 35–39. • Smartt, J. (Editor), 1994. The groundnut crop: a scientific basis for improvement. Chapman and Hall, London, United Kingdom. 734 pp. • Stalker, H.T., 1997. Peanut (Arachis hypogaea L.). Field Crops Research 53: 205–217. • Wynne, J.C., Beute, M.K. & Nigam, S.N., 1991. Breeding for disease resistance in peanut. (Arachis hypogaea L). Annual Review of Phytopathology 29: 279–303. | |||
| show more data (103) | comments (0) |
| • Burkill, H.M., 1995. The useful plants of West Tropical Africa. 2nd Edition. Volume 3, Families J–L. Royal Botanic Gardens, Kew, Richmond, United Kingdom. 857 pp. • Clavel, D., 2002. Biotechnologies et arachide. Oléagineux, Corps Gras, Lipides 9(4): 206–211. • Clavel, D. & Gautreau, J., 1997. L’arachide. In: Charrier, A., Jacquot, M., Hamon, S. & Nicolas, D. (Editors). L’amélioration des plantes tropicales. Centre de coopération internationale en recherche agronomique pour le développement (CIRAD) & Institut français de recherche scientifique pour le développement en coopération (ORSTOM), Montpellier, France. pp. 61–82. • de Waele, D. & Swanevelder, C.J., 2001. Groundnut. In: Raemaekers, R.H. (Editor). Crop production in tropical Africa. DGIC (Directorate General for International Co-operation), Ministry of Foreign Affairs, External Trade and International Co-operation, Brussels, Belgium. pp. 747–763. • Gillett, J.B., Polhill, R.M., Verdcourt, B., Schubert, B.G., Milne-Redhead, E., & Brummitt, R.K., 1971. Leguminosae (Parts 3–4), subfamily Papilionoideae (1–2). In: Milne-Redhead, E. & Polhill, R.M. (Editors). Flora of Tropical East Africa. Crown Agents for Oversea Governments and Administrations, London, United Kingdom. 1108 pp. • ILDIS, 2005. World database of Legumes, Version 9,00. International Legume Database & Information Service. [Internet] http://www.ildis.org/. Accessed June 2005. • Isleib, T.G. & Wynne, J.C., 1992. Use of plant introductions in plant improvement. In: Shads, H.L. & Weiser, L.E. (Editors). Use of plant introductions in cultivar development. Part 2. CSSA Special Publication No 20. Crop Science Society of America, Madison, Wisconsin, United States. pp. 77–116. • Kochert, G., Stalker, H.T., Gimenes, M., Galgaro, L., Romero Lopes, C. & Moore, K., 1996. RFLP and cytogenetic evidence on the origin and evolution of allotetraploid domesticated peanut, Arachis hypogaea (Leguminosae). American Journal of Botany 83(10): 1282–1291. • Lynch, R.E. & Mack, T.P., 1995. Biological and biotechnical advances for insect management in peanut. In: Patte, H.E. & Stalker, H.T. (Editors). Advances in peanut science. American Peanut Research and Education Society, Stillwater, Oklahoma, United States. pp. 95–159. • McDonald, D., Reddy, D.V.R., Sharma, S.B., Mehan, V.K. & Subrahmanyam, P., 1998. Diseases of groundnut. In: Allen, D.J. & Lenné, J.M. (Editors). The pathology of food and pasture legumes. CAB International, Wallingford, United Kingdom. pp. 63–124. • Neuwinger, H.D., 2000. African traditional medicine: a dictionary of plant use and applications. Medpharm Scientific, Stuttgart, Germany. 589 pp. • Norden, A., Smith, O.D. & Gorbet, D.W., 1982. Breeding of the cultivated peanut. In: Patte, H. & Young, C. (Editors). Peanut science and technology. American Peanut Research and Education Society, Yaokum, Texas, United States. pp. 21–49. • Popelka, J.C., Terryn, N. & Higgins, T.J.V., 2004. Gene technology for grain legumes: can it contribute to the food challenge in developing countries? Plant Science 167: 195–206. • Purseglove, J.W., 1968. Tropical Crops. Dicotyledons. Longman, London, United Kingdom. 719 pp. • Sherwood, J.L., Beute, M.K., Dickson, D.W., Elliott, J.V., Nelson, R.S., Opperman, C.H. & Shew, B.B., 1995. Biological and biotechnological control advances in Arachis diseases. In: Patte, H.E. & Stalker, H.T. (Editors). Advances in peanut science. American Peanut Research and Education Society, Stillwater, Oklahoma, United States. pp. 160–206. • Singh, A.K., 1995. Groundnut. In: Smartt, J. & Simmonds, N.W. (Editors). Evolution of crop plants. 2nd Edition. Longman, London, United Kingdom. pp. 246–250. • Singh, A.K. & Nigam, S.N., 1997. Groundnut. In: Fuccillo, D., Sears, L. & Stapleton, P. (Editors). Biodiversity in trust: conservation and use of plant genetic resources in CGIAR Centres. Cambridge University Press, Cambridge, United Kingdom. pp. 114–127. • Steinman, H.A., 1996. ‘Hidden’ allergens in foods. The Journal of Allergy and Clinical Immunology 98(2): 241–250. • USDA, 2004. USDA national nutrient database for standard reference, release 17. [Internet] U.S. Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory, Beltsville Md, United States. http://www.nal.usda.gov/fnic/foodcomp. Accessed May 2005. • Wynne, J.C. & Gregory, W.C., 1981. Peanut breeding. Advances in Agronomy 34: 39–72. | |||
| show more data (69) | comments (0) |
| show more data (20) | comments (0) |
| • Shorter, R. & Patanothai, A., 1989. Arachis hypogaea L. In: van der Maesen, L.J.G. & Somaatmadja, S. (Editors). Plant Resources of South-East Asia No 1. Pulses. Pudoc, Wageningen, Netherlands. pp. 35–39. | |||
| show more data (2) | comments (0) |
| |||||
| |||||||
| |||||||
| |||||
| Ntare, B.R., 2007. Arachis hypogaea L. [Internet] Record from PROTA4U. van der Vossen, H.A.M. & Mkamilo, G.S. (Editors). PROTA (Plant Resources of Tropical Africa / Ressources végétales de l’Afrique tropicale), Wageningen, Netherlands. <http://www.prota4u.org/search.asp>. Accessed . | |||
| There are 25 study abstracts related to Arachis hypogaea L.. Click on "show more" to view them. | ||
| show more data | comments (0) |
| There are 100 book citations related to Arachis hypogaea L.. Click on "show more" to view them. | ||
| show more data | comments (0) |
| There are 101 citation in web searches related to Arachis hypogaea L.. Click on "show more" to view them. | ||
| show more data | comments (0) |
| There are 94 citation in scholarly articles related to Arachis hypogaea L.. Click on "show more" to view them. | ||
| show more data | comments (0) |
| There are 100 news article citations related to Arachis hypogaea L.. Click on "show more" to view them. | ||
| show more data | comments (0) |
| There are 20 citations in Afrirefs related to Arachis hypogaea L.. Click on "show more" to view them. | ||
| show more data | comments (0) |
| There are 37 Wikipedia citations related to Arachis hypogaea L.. Click on "show more" to view them. | ||
| show more data | comments (0) |
| General importance |  |
| Geographic coverage Africa |  |
| Geographic coverage World |  |
| Cereals and pulses |  |
| Vegetables |  |
| Forage/feed use |  |
| Medicinal use |  |
| Vegetable oil use |  |
| Stimulant use |  |
| Fibre use |  |
| Food security |  |